Following a competitive global application process, Cochrane has announced the establishment of five new Evidence Synthesis Units (ESUs) around the world. Among them, is the Australian Cochrane Evidence Synthesis Unit which includes:
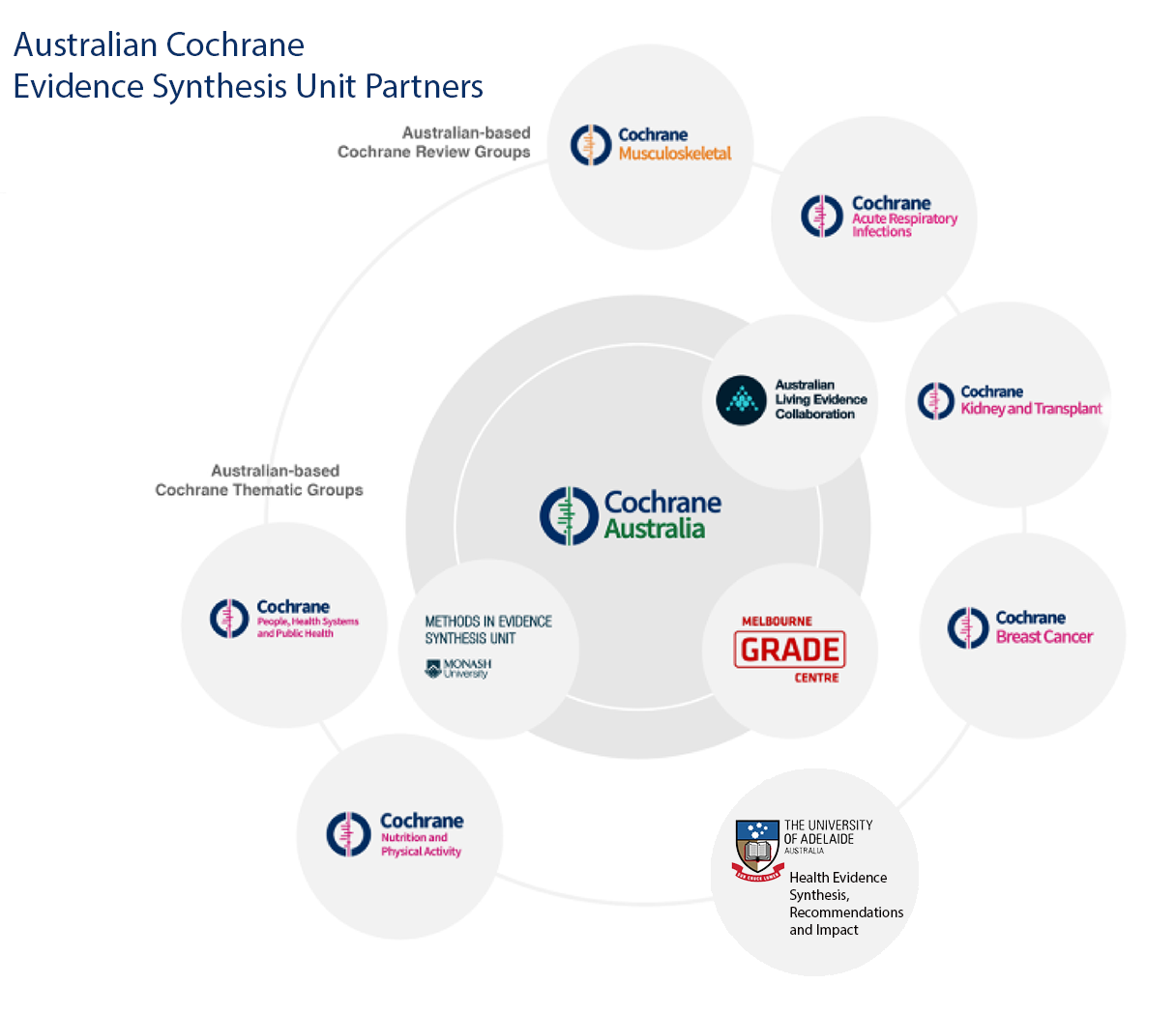
- Cochrane Australia within the School of Public Health and Preventive Medicine at Monash University (including the Australian Living Evidence Collaboration (ALEC), the Melbourne GRADE Centre and Methods in Evidence Synthesis Unit)
- Australian based Cochrane Review Groups including Breast Cancer, Kidney and Transplant, Acute Respiratory Infections and Musculoskeletal
- Australian based Thematic Groups including People, Health Systems and Public Health and Nutrition and Physical Activity
- Health Evidence Synthesis, Recommendations & Impact (HESRI) at the University of Adelaide.
‘Together with all our partners, we welcome the opportunity to establish one of the first Cochrane Evidence Synthesis Units here in Australia,’ says Cochrane Australia Co-Director Sally Green. ‘For nearly 30 years, Cochrane Australia and the Australian-based Cochrane groups have been home to many of Cochrane’s leading experts in evidence synthesis, systematic review methods and knowledge translation. We now look forward to drawing on our collective expertise, technical know-how and long-standing partnerships to build this new collaboration. We are keen to share our skills and resources to respond to the diverse needs of stakeholders nationally, regionally and globally.’
The new Australian Cochrane ESU will include research and support staff with significant individual and collective track records, not only in delivering a wide range of evidence syntheses but also in pioneering new methods and types of synthesis, developing new platforms to support evidence synthesis production, and implementing novel dissemination formats – as Dr Rebecca Ryan of Cochrane People, Health Systems and Public Health and Coordinating Editor of Cochrane Consumers and Communication explains.
‘We’re so fortunate to have Cochrane Thematic and Review Group partners across our region that are global leaders in their respective areas, with decades of experience in supporting and producing Cochrane Reviews and other types of evidence synthesis.’
‘Taken together, our ESU Partnership Network brings a wealth of clinical and methodological expertise across priority topic areas of interest to Australian governments, including chronic disease, health services, public health and women’s health. Combining our networks also opens possibilities to connect with an even wider range of stakeholders nationally and globally.’
‘The collective experience and skills of our networked groups were reflected in the substantial portfolio of commissioned work highlighted in our ESU application,’ Rebecca adds. ‘This included a variety of evidence syntheses (systematic reviews, overviews, qualitative reviews, rapid reviews, evidence maps, clinical practice guidelines) and knowledge translation outputs (decision aids, consumer resources, podcasts, videos, infographics and media coverage) – along with significant expertise in systematic reviews methods and GRADE training. It’s a really comprehensive offering by a committed and talented network of experts who have worked collaboratively over many years.’
The independent panel assessing the ESU applications appears to have similarly valued this experience and collaborative potential.
‘Following a rigorous and very competitive selection process, we are confident that our new pilot Evidence Synthesis Units in Australia, Iberoamerica, India, Germany and Nigeria will adopt innovative methods, promote health equity and champion research integrity and partnerships to support Cochrane’s long-term sustainability,’ Cochrane’s Editor in Chief, Dr Karla Soares-Weiser said when announcing the successful bids in early June.
‘The geographic spread of the new units reflects Cochrane’s commitment to producing evidence that helps to improve people’s health worldwide. It’s part of our wider Future of Evidence Synthesis programme that aims to transform Cochrane’s ability to deliver reviews that respond to the different needs of our users worldwide. By implementing structural changes, we aim to be agile in an incredibly competitive environment, to deliver on our new Scientific Strategy, and to get the high quality evidence for which Cochrane is recognised into the hands of those who need it – wherever they live and work around the globe.’
Cochrane Australia and all our partners look forward to contributing to these goals as a new pilot ESU in the months and years ahead.
